Neat Info About How To Draw Lewis Dot Structures

Count total valence electrons for the molecule.
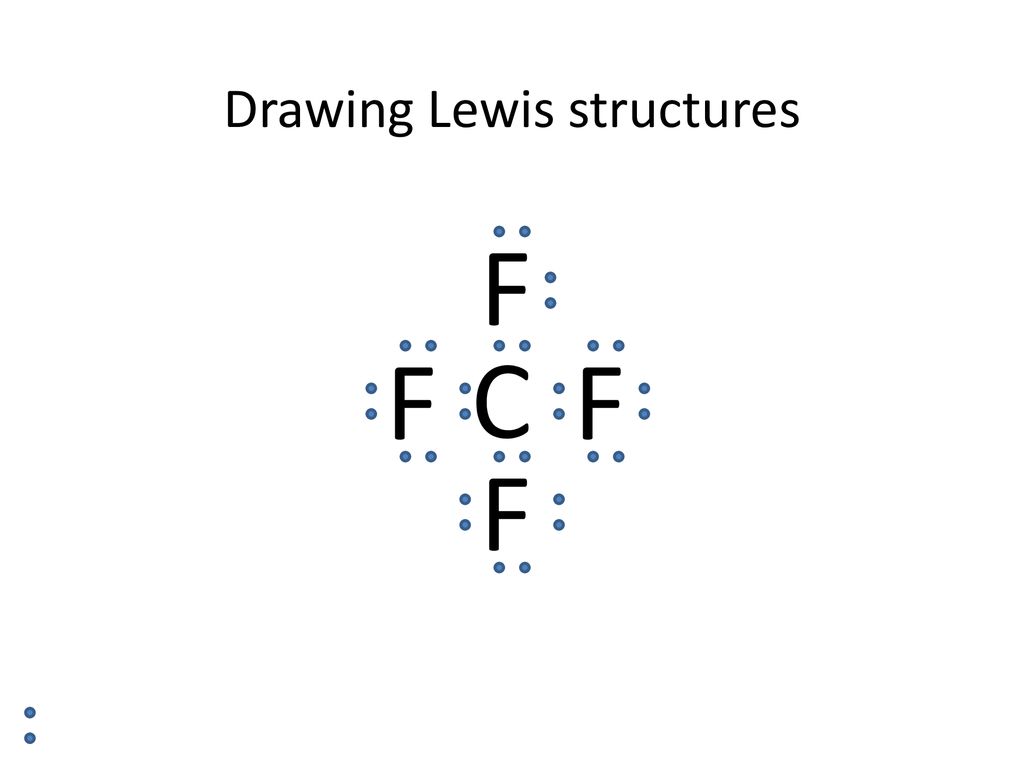
How to draw lewis dot structures. Calculate the valence electrons present at each atom in the given molecule. For a neutral molecule or an atom, to draw a lewis dot diagram, follow the given steps: The maximum number of dots can be eight.
This video shows you how to draw lewis dot structure in 5 easy steps. The dots should be neatly drawn on the four sides of the square with no more than two electrons on each side. How to create a lewis dot structure.
Set up skeleton with single bonds (central atom is the atom which can make the most bonds). 8.2 lewis dot structures 1. Put the least electronegative atom in the center with other atom.
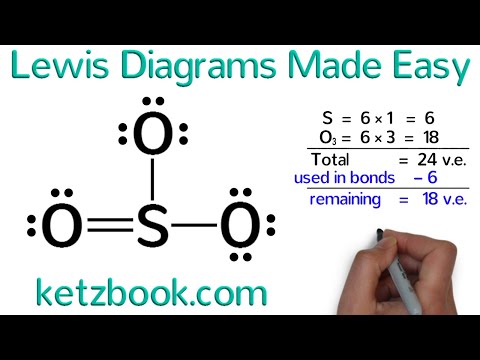
This chemistry video provides a basic introduction into how to draw lewis structures of common molecules such as cl2, o2, of2, ch4, nh3, h2o, c2h2, and n2h4. 8.2 how to draw lewis dot structures | complete guide | general chemistry. Fill the octets of the outside atoms with lone pairs of electrons.
To draw the lewis structure, you will need to know the total number of valence electrons present. Lewis dot structure of polyatomic ions. The lewis structure is drawn for individual atoms by putting a dot for each available valence electron around the atom.
This is because the maximum. Find the total number of valence electrons.





/Lewis-dot-structure-58e5390f3df78c5162b4c3db.jpg)