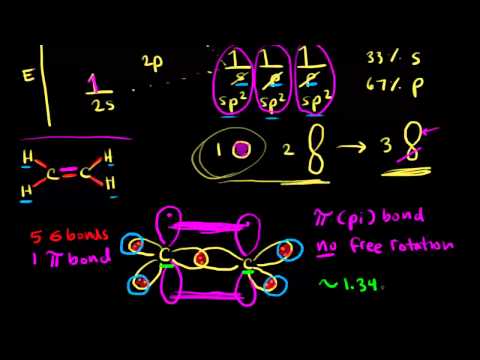
Peerless Info About How To Draw Hybrid Orbitals

They have the same shape and energy (are.
How to draw hybrid orbitals. To draw the orbital diagram for an atom, follow these basic steps. This organic chemistry video tutorial provides a basic introduction into valence bond theory and hybrid atomic orbitals. For h3o+ oxygen has 6 valence electrons, 3 hydrogens have three, minus one electron equals 8.
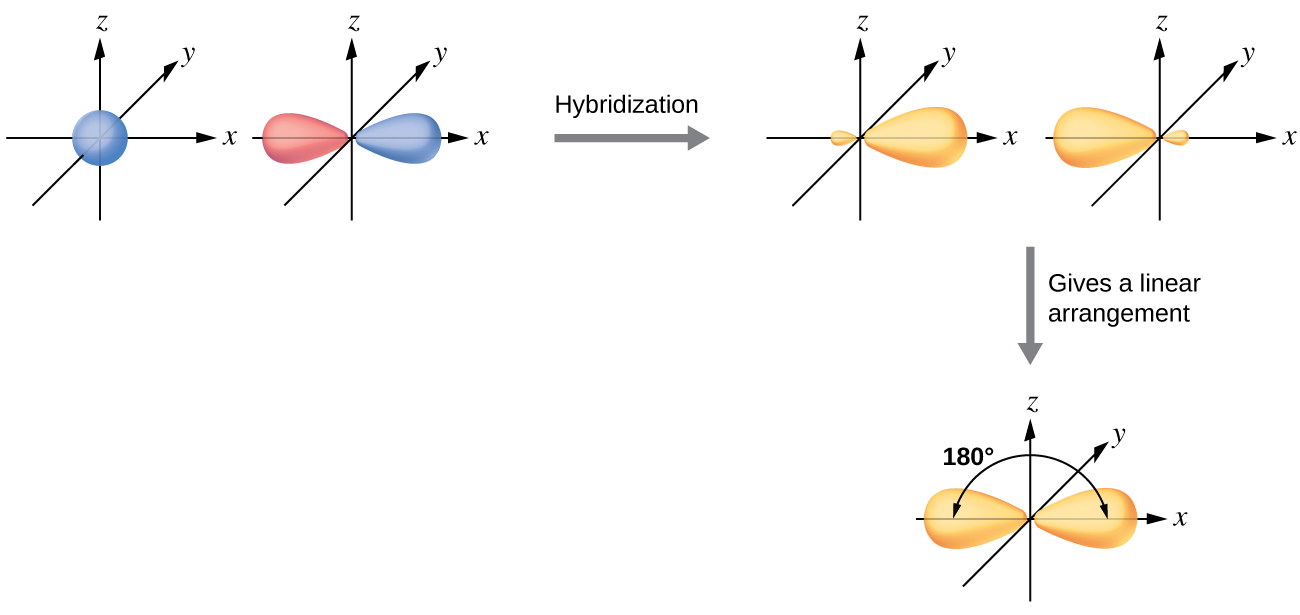
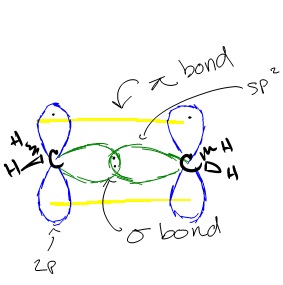
Hybridization of an s orbital (blue) and a p orbital (red) of the same atom produces two sp hybrid orbitals (purple). Nevertheless, there is a general strategy for finding orbital shapes for the simpler cases. Line joining the two hybrid sp orbitals.
It explains how to find the hybridi. Decide how many orbitals each atom needs to make its sigma bonds. One way to predict hybrid orbitals is to calculate the number of electron pairs.
Procedure for constructing molecular orbital diagrams based on hybrid orbitals 1. When we write 2 x sp we mean two instances of sp and when we write 2p we mean one instance of a. 2s 2p sp sp 2 x sp 2 x sp + 2p 2 x sp + 2 x 2p note:
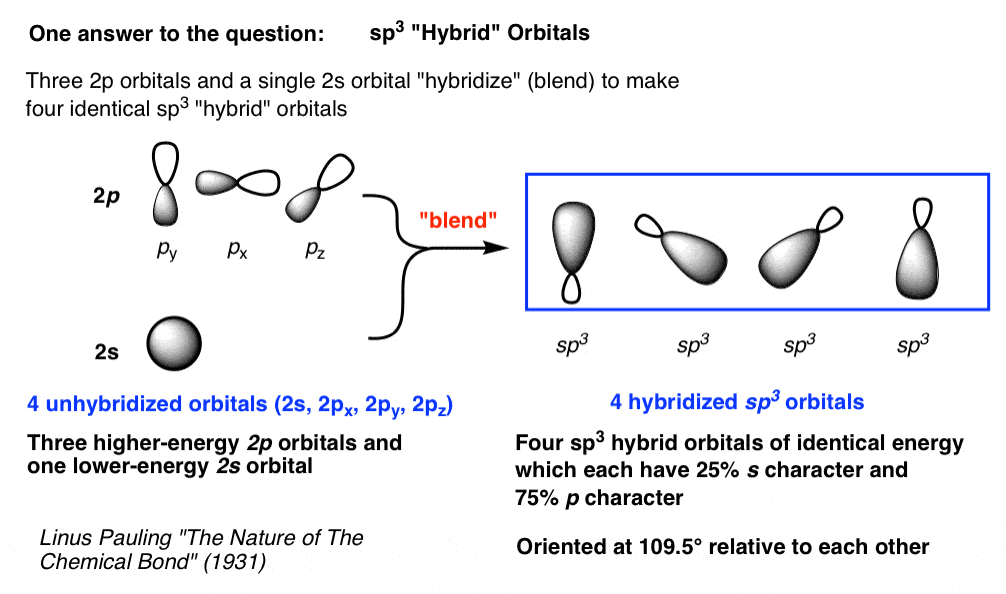
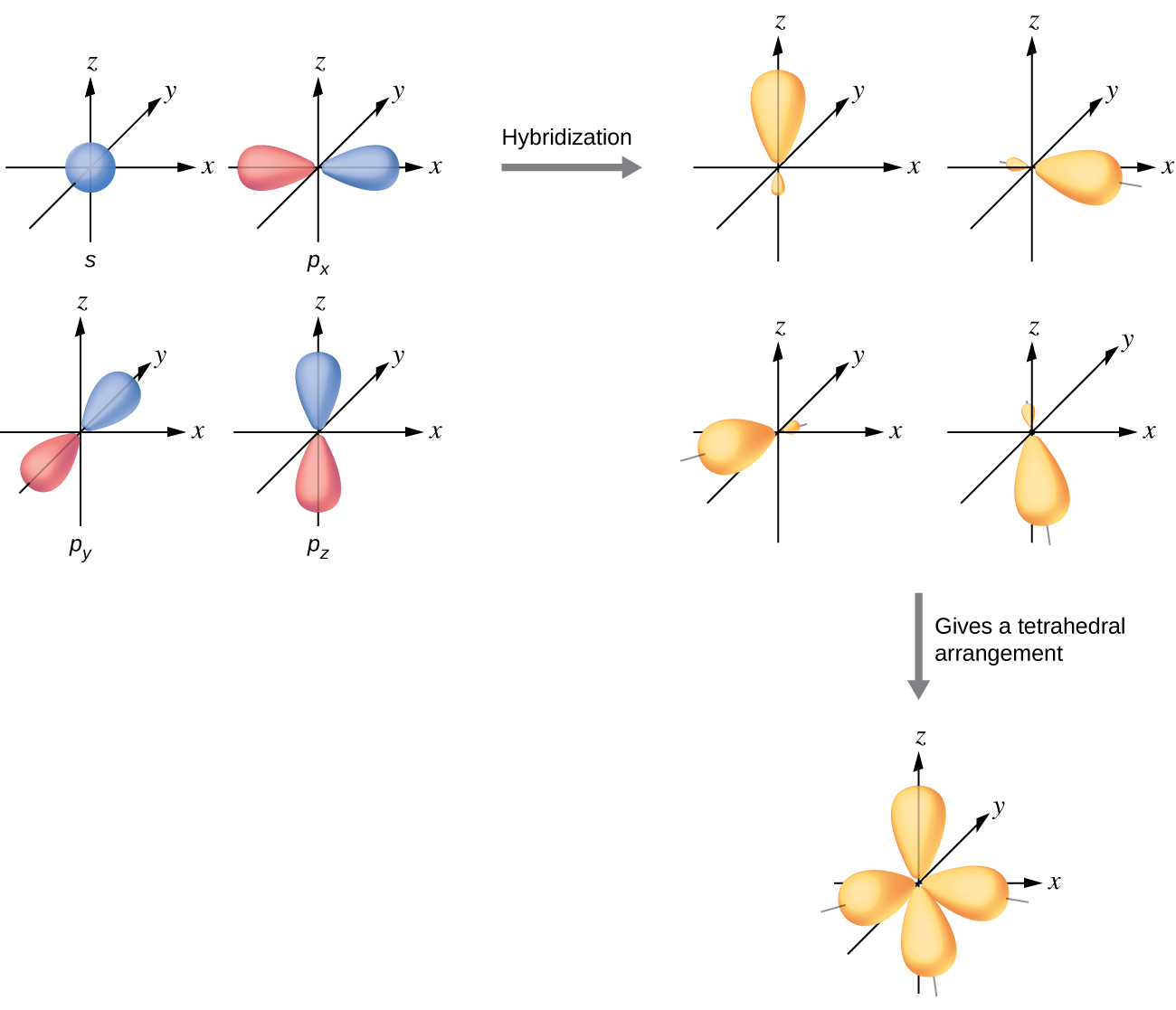
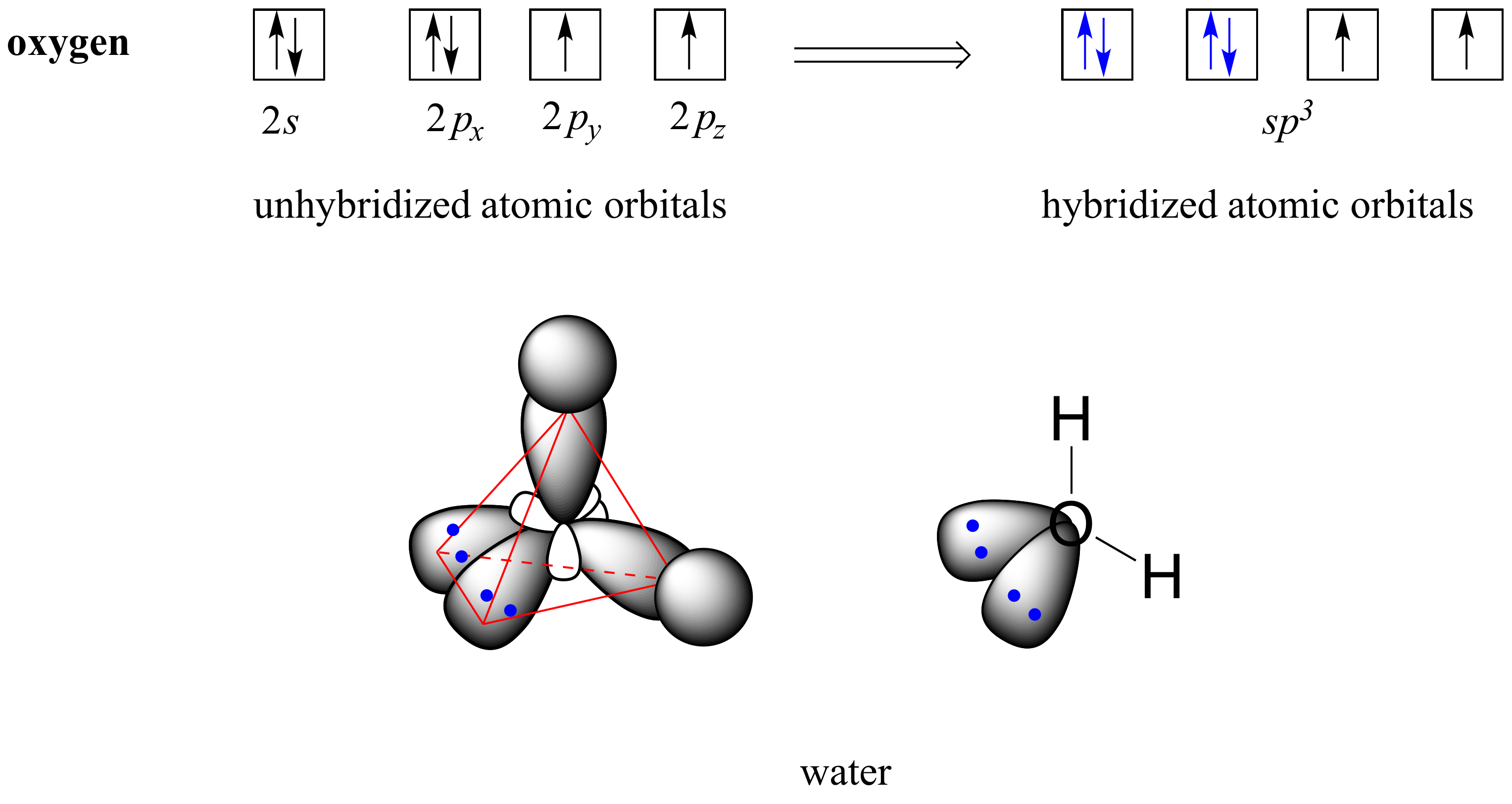
To get four new orbitals we have to combine four atomic orbitals. Different hybrid orbitals have different shapes, so there is no simple procedure for deriving all of them. The four sp 3 hybrid atomic orbitals are formed from the combination of the 2s atomic orbital with all three 2p atomic orbitals.
Each hybrid orbital is oriented primarily in just one direction. Find the number of electrons in an atom. Write the electron configuration for an atom to determine which orbitals should be.







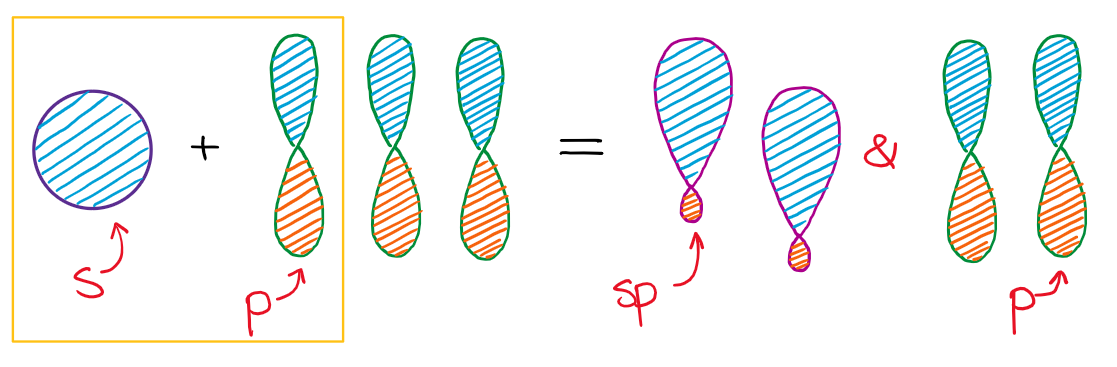



/hybrid-orbital-38d0c0f547ef46268127f68ce20714fa.jpg)